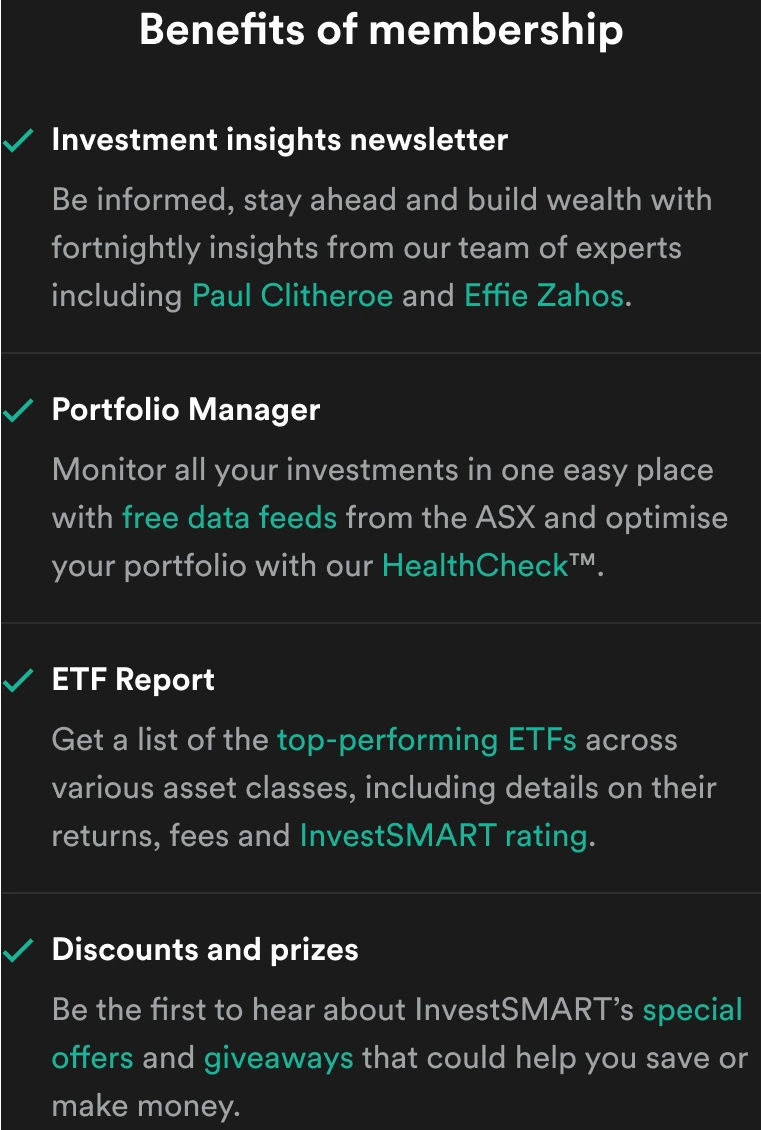
FDA rejects Pharmaxis drug push bid
Australian biotech company Pharmaxis' quest for profit has been delayed again, after its bid to market the cystic fibrosis drug Bronchitol in the US was rejected by the main regulatory body.
Australian biotech company Pharmaxis' quest for profit has been delayed again, after its bid to market the cystic fibrosis drug Bronchitol in the US was rejected by the main regulatory body.
The US Food and Drug Administration said in a letter to Pharmaxis on Tuesday that it could not yet approve Bronchitol, and recommended an additional trial.
"The submitted data do not provide a favourable benefit-risk balance to support the use of inhaled mannitol in patients with cystic fibrosis six years of age and older," the FDA said.
Pharmaxis chief executive Gary Phillips, who replaced CEO Alan Robertson last week in a management shake-up, said the drug maker was "clearly disappointed" and it would be taking part in a follow-up meeting with the FDA.
"The company remains committed to bringing Bronchitol to [cystic fibrosis] patients in the US and the onus is now on Pharmaxis to work with the FDA to ensure Bronchitol is approved as soon as possible," Mr Phillips said.
Shares in Pharmaxis closed 7.1per cent down at 46¢ on Tuesday.
Mr Phillips' appointment to the top job had reflected a shift by Pharmaxis away from development towards the commercialisation of its drugs, and followed a series of setbacks.
In January, a negative review of Bronchitol by advisers to the FDA saw shares in the pharmaceutical firm plunge more than 45 per cent in one day.
The FDA rejection was not unexpected, with analysts pointing to the coming results of Pharmaxis' Phase III trial of Bronchitol in patients with bronchiectasis - completed in early March - as key to the firm's outlook.
"They don't need another slip-up. There is a fair bit weighing on the Phase III results," RBS Morgans healthcare and biotechnology analyst Scott Power said.
Shaw Stockbroking analyst Darren Vincent said bronchiectasis had a larger patient population than cystic fibrosis, and a successful trial could see Pharmaxis obtain label extensions for the marketing of the drug in Europe and Australia.
"While what's happened with the FDA represents a lot of disappointment and the share price has been caned for it, it's not game over by any sense," Mr Vincent said.
Mr Vincent said a $US40million ($38.5million) financing agreement Pharmaxis signed with NovaQuest Pharma Opportunities Fund III in January was expected to cover the costs of an additional Bronchitol trial.
Pharmaxis reported a $20.8million net loss for the six months to December, a 6per cent rise from the previous corresponding period.
The US Food and Drug Administration said in a letter to Pharmaxis on Tuesday that it could not yet approve Bronchitol, and recommended an additional trial.
"The submitted data do not provide a favourable benefit-risk balance to support the use of inhaled mannitol in patients with cystic fibrosis six years of age and older," the FDA said.
Pharmaxis chief executive Gary Phillips, who replaced CEO Alan Robertson last week in a management shake-up, said the drug maker was "clearly disappointed" and it would be taking part in a follow-up meeting with the FDA.
"The company remains committed to bringing Bronchitol to [cystic fibrosis] patients in the US and the onus is now on Pharmaxis to work with the FDA to ensure Bronchitol is approved as soon as possible," Mr Phillips said.
Shares in Pharmaxis closed 7.1per cent down at 46¢ on Tuesday.
Mr Phillips' appointment to the top job had reflected a shift by Pharmaxis away from development towards the commercialisation of its drugs, and followed a series of setbacks.
In January, a negative review of Bronchitol by advisers to the FDA saw shares in the pharmaceutical firm plunge more than 45 per cent in one day.
The FDA rejection was not unexpected, with analysts pointing to the coming results of Pharmaxis' Phase III trial of Bronchitol in patients with bronchiectasis - completed in early March - as key to the firm's outlook.
"They don't need another slip-up. There is a fair bit weighing on the Phase III results," RBS Morgans healthcare and biotechnology analyst Scott Power said.
Shaw Stockbroking analyst Darren Vincent said bronchiectasis had a larger patient population than cystic fibrosis, and a successful trial could see Pharmaxis obtain label extensions for the marketing of the drug in Europe and Australia.
"While what's happened with the FDA represents a lot of disappointment and the share price has been caned for it, it's not game over by any sense," Mr Vincent said.
Mr Vincent said a $US40million ($38.5million) financing agreement Pharmaxis signed with NovaQuest Pharma Opportunities Fund III in January was expected to cover the costs of an additional Bronchitol trial.
Pharmaxis reported a $20.8million net loss for the six months to December, a 6per cent rise from the previous corresponding period.
Share this article and show your support